There are a variety of reasons that could trigger a drug recall, such as contamination, fluctuations in potency, erroneous labeling, or inefficacy. Alarmingly, certain drugs may even be withdrawn due to intolerable side effects, which would make their continued sales ethically problematic. It’s a common perception that if the Food and Drug Administration, or FDA, approves a product, it is safe, thoroughly examined and all potential side effects have been assessed. But this isn’t always the case. Look at these 25 Promising Drugs That Received FDA Approval But Were Subsequently Recalled as proof.
Feature Image: Shutterstock
Diethylstilbestrol
 Source: http://247wallst.com/
Source: http://247wallst.com/ Also known as DES, this drug was recalled in 1975 after nearly forty years on the market. A drug intended to prevent miscarriage, it was found years later to cause tumors in DAUGHTERS of the women whose mothers had taken it while pregnant. It is still sometimes prescribed to men to treat estrogen deficiencies though.
Cylert
 Source: http://prescriptiondrugs.procon.org/
Source: http://prescriptiondrugs.procon.org/ Cylert was a drug that affected the central nervous system and was used to treat ADD/ADHD. It was on the market for 35 years before it was pulled in 2010 because it caused liver damage.
Dextropropoxyphene
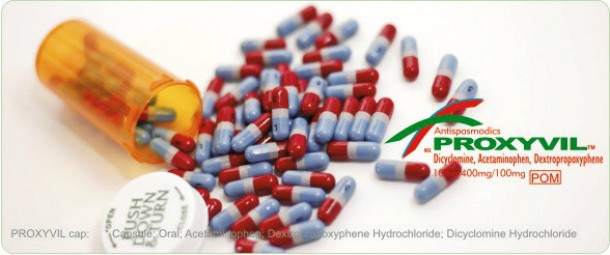 Source: https://en.wikipedia.org
Source: https://en.wikipedia.org This drug was used as a mild pain reliever and sometimes cough suppressant. It also had mild local anesthetic effects. It was taken off the market in the US in 2010 and in Europe on 2009 due to heart arrhythmia and fatal overdoses.
Darvon & Darvocet
 Source:http://www.webmd.com/
Source:http://www.webmd.com/ After being on the market for over half a century, Darvon & Darvocet were banned in 2010 in the US, six years after being banned in the UK. Specifically, the ingredient propoxyphene was banned, due to heart side effects such as fatal abnormal rhythms in otherwise healthy patients taking the drugs as directed.
Cerivastatin (Baycol)
 Source: http://247wallst.com/
Source: http://247wallst.com/ Cerivastatin (also known as Baycol) was recalled after just four years on the market in 2001. Intended to treat high cholesterol, it caused severe kidney and muscle issues and is responsible for at least 52 deaths.
Bromfenac (Duract)
 Source: http://prescriptiondrugs.procon.org/
Source: http://prescriptiondrugs.procon.org/ Bromfenac (also known as Duract) was a non-steroidal anti-inflammatory drug (or NSAID) that was pulled from the market after just ONE year in 1998 because despite directions clearly stating to only use for ten days or less, people took it for more than ten days, and it caused severe liver damage that in same cases required transplant or resulted in death.
Lotronex (Alosetron)
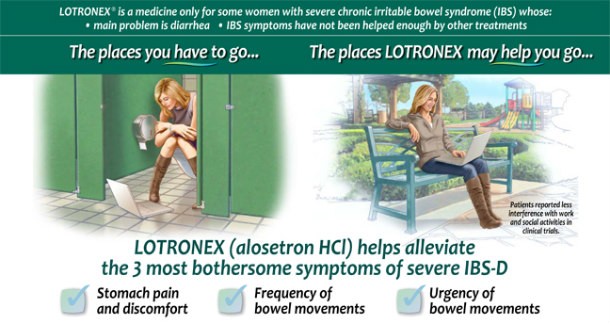 SourceLhttp://www.nytimes.com/
SourceLhttp://www.nytimes.com/ Lotronex (also known as Alosetron) was released on the market and recalled the same year, 2000. A drug that was used to treat Irritable Bowel Syndrome, Lotronex also has some major side effects such as intestinal inflammation and injury and resulted in 5 deaths. The interesting thing about Lotronex is that patients who had been greatly helped by the drug complained loudly to the FDA about the recall, as many of them had suffered no ill side effects. Due to this, the drug was reintroduced in 2002, with restricted use and more patient observation.
Pergolide
 Source: https://en.wikipedia.org
Source: https://en.wikipedia.org This drug, also known as Permax and Prascend, was a drug used to treat Parkinson’s patients and was on the market for nearly two decades before being recalled in 2007 due to causing damage to heart valves. It’s still available to treat Cushing’s disease in horses, however. Some patients in Australia who took the drug claimed it caused gambling and sex addictions (which is actually possible considering it worked on dopamine receptors in the brain).
Encainide (Enkaid)
 Source: http://ahrp.org/
Source: http://ahrp.org/ Encainide (also know as Enkaid) was used to treat irregular heart rhythms but was pulled from the market in 1991 due to causing proarrhythmic side effects, which is a fancy way of saying the medicine used to treat abnormal rhythms of the heart eventually caused more…abnormal rhythms of the heart.
Mylotarg
 Source: http://www.fda.gov/
Source: http://www.fda.gov/ Lukemia treatment Mylotarg was voluntarily recalled by manufacturer Pfizer at the request of the FDA after a decade on the market due to there being no improvement shown in trials where Mylotarg was added to chemotherapy treatments, and the groups of patients who received Mylotarg had a higher instance of death.
Propulsid (Cisapride)
 Source: https://en.wikipedia.org
Source: https://en.wikipedia.org Propulsid (also known as Cisapride) was used to treat GERD and pulled from the market in 2000 after less than ten years on the market due to causing severe cardiac arrhythmias.
Vioxx
 Source:http://prescriptiondrugs.procon.org/
Source:http://prescriptiondrugs.procon.org/ Vioxx was an NSAID recalled in 2004 after 5 years on the market. It was linked to over 27 THOUSAND sudden cardiac arrests or cardiac deaths.
Terfenadine (Seldane)
 Source:http://247wallst.com/ Image Source: pixabay.com (public domain)
Source:http://247wallst.com/ Image Source: pixabay.com (public domain) Terfenadine (also commonly known as Seldane) was recalled in 1997 after 13 years on the market. It was a non-drowsy allergy drug that unfortunately caused heart arrhythmia when taken with other drugs. Over 100 million people used Terfenadine at the time of it’s recall, making it one of the largest recalls on this list. Aventis, the company that made the drug, introduced Allerga soon after.
Bextra (Valdecoxib)
 Source: http://www.drugsdb.com/
Source: http://www.drugsdb.com/ Bextra (also known as Valdecoxib) was a NSAID used to treat arthritis, designed to have less stomach side effects. It was pulled in 2005, after a massive amount of lawsuits due to its users being more than twice as likely to experience blood clots, stroke, heart attacks, and a deadly skin condition.
DBI (Phenformin)
 Source: http://prescriptiondrugs.procon.org/
Source: http://prescriptiondrugs.procon.org/ DBI ( also known as Phenformin) was an anti-diabetic drug that was on the market for almost two decades before it was pulled in the late 70’s. It caused a buildup of lactic acid and low ph in tissues of patients taking the drug.
Rapacuronium (Raplon)
 Source: https://en.wikipedia.org
Source: https://en.wikipedia.org Rapacuronium (also known as Raplon) was a drug used during general anesthesia to assist with incubation. It was withdrawn in 2001 after less than two years on the market due to risk of fatal bronchialspasm associated with use of the drug.
FEN-PHEN
 Source: http://www.drugsdb.com/
Source: http://www.drugsdb.com/ FEN-PHEN (also known as fenfluramine/dexfenfluramine and phentermine) was a combination weight loss drug, and it worked reportedly very well. Unfortunately, it also caused heart valve issues and sometimes death in the women who took it.
Mibefradil (Posicor)
 Source: http://247wallst.com/
Source: http://247wallst.com/ Mibefradil (also know as Posicor) was recalled after just a single year on the market in 1998 after being linked to 123 deaths. Intended to treat high blood pressure and angina, Posicor had deadly interactions when combined with other, common drugs.
Zelmid
 Source: http://ahrp.org/
Source: http://ahrp.org/ Zelmid was a anti-depressant that was both approved and recalled by the FDA in 1982. The FDA literally pulled the plug on this drug before it reached market because it actually caused a higher risk of suicide. Thank you FDA, but also, how did that get approved in the first place, FDA?
Trasylol
 Source: http://prescriptiondrugs.procon.org/
Source: http://prescriptiondrugs.procon.org/ Trasylol first came into use in the 1960’s and was pulled from the market in 2008. It was used to reduce blood loss during surgery but had serious side effects such as kidney damage, heart failure, strokes, and increased risk of death.
Selacryn
 Source: http://www.nytimes.com/
Source: http://www.nytimes.com/ Selacryn was a blood pressure medication recalled in the early 1980’s after less than a decade on the market, due to hepatitis, over 30 deaths, and hundreds of cases of severe kidney and liver damage. SmithKline, the drug’s manufacturer, pleaded guilty in court to not disclosing the known side effects to the FDA in order to get the drug to market. Shame on them.
If you’ve enjoyed this list, check out 25 Curious Facts About Cannabis Most People Don’t Realize.
Quaalude
 Source: http://ahrp.org/
Source: http://ahrp.org/ Quaalude, which was marketed under many different names and sometimes used as a date rape drug, is a sedative and was pulled off the market in 1985 due to fun side effects like mania, seizures, and death. It is now listed as a Schedule I drug in the US, like LSD and Heroine, meaning no prescriptions can be written for it, and the FDA believes the drug has little to no therapeutic value.
Meridia (Sibutramine)
 Source: http://www.fda.gov/
Source: http://www.fda.gov/ Meridia (also known as Sibutramine) was an appetite suppressing drug used to treat obesity that was on the market for 13 years before being pulled in 2010 due to increased heart attack and stroke risk. You know..the same things that being obese puts you at higher risk for.
Hismanal (Astemizole)
 Source:http://prescriptiondrugs.procon.org/
Source:http://prescriptiondrugs.procon.org/ Hismanal an antisphycotic (also known as Astemizole) was withdrawn from the US market in 1999 after over a decade of use, due to its effect on potassium channels in the heart.
Accutane
 Source: http://prescriptiondrugs.procon.org/ Image Source: commons.wikimedia.org (public domain)
Source: http://prescriptiondrugs.procon.org/ Image Source: commons.wikimedia.org (public domain) Accutane (a form of vitamin A) was a very popular acne drug for nearly thirty years before it was pulled from the market in 2009 due to side effects including: suicidal tenancies, irritable bowl syndrome, and a whole host of issues for women who later became pregnant such as miscarriage, premature birth, and severe birth defects. However, there are generic brands of the medication that are surprisingly still available on the market.
Photos: 25. DES Daughter via Flickr, 24. Cylert info sheet (fair use), 23. Proxyvil (with Dextropropoxyphene – fair use – demonstrative purposes only), 22. Darvocet (fair use), 21. BruceBlaus via wikimedia commons, 20. BruceBlaus via wikimedia commons, 19. Lotronex info sheet (fair use), 18. Pergolide (fair use), 17. Encainide (fair use), 16. Mylotarg (fair use), 15. Propulsid (fair use), 14. David Jordan via wikimedia commons, 12. Bextra (fair use), 11. Phenformin (fair use), 10. Raplon (fair use), 9. FEN-PHEN (fair use), 8. Ian Furst via wikimedia commons, 7. Sander van der Wel via Flickr, 6. Trasylol (fair use), 5. www.volganet.ru via wikimedia commons, 4. Quaalude (fair use), 3. Meridia (fair use), 2. Hismanal (fair use)



























